Background: Natural killer (NK) cells are part of the innate immune system, and their killing capacity depends on the inputs from activating and inhibitory receptors. CD226 is an activating receptor that plays a role in the immune surveillance of acute myeloid leukemia (AML). AML cells have developed mechanisms to escape NK cell cytotoxicity including downregulating activating receptors such as CD226 on the surface of NK cells. Chimeric antigen receptors (CARs) are synthetic receptors integrated into NK cells to direct their anti-leukemia activity, and CD38 is a potential target for NK CAR-based cellular therapy against AML. Understanding the dynamics between the endogenous activating and inhibitory NK cell receptors (such as CD226) and the introduced CAR can determine the development of more effective NK CAR-based therapy for AML. We hypothesize that CD38-directed CAR NK cells may overcome CD226 downregulation as a mechanism of immune escape in AML.
Purpose: we aimed to investigate (1) the mechanisms developed by AML cells to escape recognition by the activating receptor CD226, (2) whether and how anti-CD38 CAR-armed NK cells can overcome AML immune escape mechanisms.
Methods: we performed multiparametric mass spectrometry to characterize changes in CD226 expression in NK cells of individuals with AML. Then, we investigated CD226 expression in NK cell-AML cocultures to characterize CD226-related mechanisms by imaging flow cytometry. Finally, to explore the impact of CD226 knockout (KO) together with the role of CD38 CAR in NK cell functionality, we retrovirally transduced CD38 CAR combined with CRISPR-Cas9 CD226 KO followed by short- and long-term cytotoxicity assays, cytokine production techniques and immune synapse assays by imaging flow cytometry.
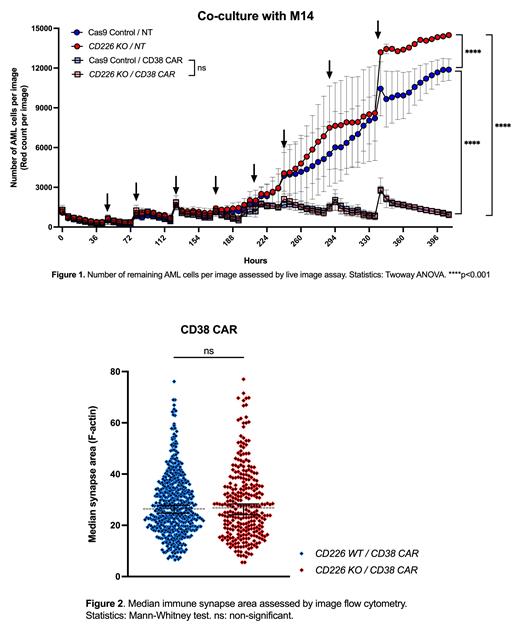
Results: NK cells from AML patients expressed low levels of CD226 with impaired cytotoxicity compared to heathy donor NK cells. Co-culture of healthy donor NK cells with AML blasts resulted in CD226 downregulation secondary to its internalization and was dependent on expression of the CD226 ligand CD155 in AML cells. CD226 KO diminished NK cell cytokine production and decreased their killing capacity against AML cells. In contrast, CD38-targeting NK cells displayed enhanced and specific cytotoxic activity against primary AML cells. When we combined the CD38 CAR with a CD226 KO in NK cells, these cells maintained their activating potential, cytokine secretion levels, and anti-leukemic cytotoxicity (Figure 1). CD38 CAR / CD226 KO NK cells could build strong immune synapses (Figure 2) even in the absence of LFA-1, an integrin activated by CD226 signaling and essential for NK cell immune synapse formation, suggesting that the CAR molecule compensates for the absence of CD266-mediated LFA signaling. Furthermore, CD38 CAR NK cells were capable of forming immune synapses, that were at least partially independent from LFA-1.
Conclusion: CAR-directed NK cells can surpass resistance mechanisms in AML, such as CD226 downregulation and dampening of LFA-1 recruitment into the immunological synapse. Thus, NK cell activation by artificially introduced and germline-encoded receptors can overcome distinct tumor-resistance mechanisms, CAR-driven NK cells being a potent option for the treatment of AML.
Disclosures
Banerjee:Takeda: Patents & Royalties, Research Funding. Lin:Takeda: Patents & Royalties, Research Funding. Daher, MD:Takeda: Patents & Royalties. Rafei:Takeda: Other: H. R. and The University of Texas MD Anderson Cancer Center have an institutional financial conflict of interest with Takeda Pharmaceutical. . Rezvani:Affimed: Other: License agreement; Takeda: Patents & Royalties.